-
Corcept Therapeutics Observes Large Reductions of Liver Fat and Transient Liver Enzyme Elevations in Phase 2 Trial of Miricorilant as a Potential Treatment for Patients with Nonalcoholic Steatohepatitis (NASH)
Источник: Nasdaq GlobeNewswire / 06 май 2021 15:05:03 America/Chicago
MENLO PARK, Calif., May 06, 2021 (GLOBE NEWSWIRE) -- Corcept Therapeutics Incorporated (NASDAQ: CORT), a commercial-stage company engaged in the discovery and development of drugs to treat severe metabolic, oncologic and psychiatric disorders by modulating the effects of cortisol, today announced interim findings from the Phase 2 trial of its selective cortisol modulator miricorilant in patients with presumed NASH.
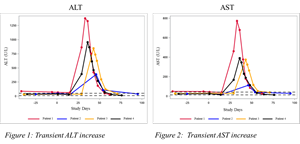
“This was our first trial to evaluate miricorilant as a treatment for patients with liver disease. When we observed elevated ALT and AST levels in four of the first five patients who received miricorilant for four weeks, we suspended the study,” said Andreas Grauer, MD, Corcept’s Chief Medical Officer. “Our investigation has made two notable findings. First, the elevations in ALT and AST resolved after miricorilant was withdrawn.” (See Figures 1 and 2)
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/00d4659c-26e2-4ecf-aa44-10cefb13a5d3
“Second, the patients with elevated liver enzymes exhibited large reductions in liver fat rapidly.” (See Table 1)
Patient Miricorilant
(per day)Days
on Drug% Liver Fat
at Baseline% Liver Fat
at Follow upDays Between
Last Dose and
Follow-upRelative
Reduction in
% Liver FatPatient 1 900 mg 30 17.6 6.1 19 -65.3 % Patient 2 900 mg 31 27.8 17.1 64 -38.5 % Patient 3 900 mg 44 28.3 15.0 16 -47.0 % Patient 4 600 mg 34 12.6 3.3 21 -73.8 % Table 1: Reduction in liver fat content measured by magnetic resonance imaging-proton density fat fraction (MRI-PDFF) “We had planned to enroll 120 patients in this study, which we thought would be sufficient to detect a 30 percent reduction in liver fat after 12 weeks,” continued Dr. Grauer. “These patients exhibited much larger reductions after receiving miricorilant for 4-6 weeks. These findings are particularly striking because their follow-up liver MRI was some distance from their final dose.”
“Liver fat reductions achieved this quickly and of this magnitude are rarely seen ,” said Dr. Stephen A. Harrison, M.D., Medical Director for Pinnacle Clinical Research, San Antonio, Texas, Visiting Professor of Hepatology, Oxford University and principal investigator in Corcept’s Phase 2 trial. “Fatty liver disease and NASH afflict many millions of people and there are no approved treatments. Further study is warranted to evaluate if miricorilant can produce such significant reductions in liver fat safely and that the reduction results in a meaningful improvement in NASH.”
About the Phase 2 Trial
This double-blind, placebo-controlled Phase 2 study is designed to enroll 120 patients with presumed NASH at 15 clinical sites in the United States. Patients are randomized 1:1:1 to receive a daily dose of 600 mg of miricorilant, 900 mg of miricorilant or placebo. The trial’s primary endpoint is reduction in liver fat at week 12 as assessed by MRI-PDFF, with a secondary endpoint being the proportion of patients who exhibit liver fat reduction of at least 30 percent at week 12. Additional information about the study (NCT03823703) is at www.ClinicalTrials.gov.
About Corcept Therapeutics
Corcept is a commercial-stage company engaged in the discovery and development of drugs to treat severe metabolic, oncologic and psychiatric disorders by modulating the effects of the stress hormone cortisol. Korlym® was the first drug approved by the U.S. Food and Drug Administration for patients with Cushing’s syndrome. Corcept has discovered a large portfolio of proprietary compounds, including miricorilant, that selectively modulate the effects of cortisol. The company owns extensive United States and foreign intellectual property covering the composition of its selective cortisol modulators and the use of cortisol modulators to treat a variety of serious disorders.
Forward Looking Statements
Statements in this press release, other than statements of historical fact, are forward-looking statements based on our current plans and expectations that are subject to risks and uncertainties that might cause our actual results to differ materially from those statements express or imply. These risks and uncertainties include, but are not limited to, the progress, enrollment, timing, design and results of our clinical trials; our ability to operate our business and achieve our goals and conduct our clinical trials during the COVID-19 pandemic; the availability of competing treatments; risks related to the development of miricorilant as a product candidate for NASH, including its clinical attributes, regulatory approvals, mandates and oversight, and other requirements; and the scope and protective power of our intellectual property. In this press release, forward-looking statements include those concerning the clinical attributes of miricorilant and its effects in patients with NASH and the requirements to resume its development as a treatment for fatty liver disease and NASH. These and other risks are set forth in our SEC filings, which are available at our website and the SEC’s website. We disclaim any intention or duty to update forward-looking statements made in this press release.
CONTACT:
Corcept Therapeutics
Investor Relations
ir@corcept.com
www.corcept.com